Itekhnoloji yokudibanisa efana ne-peptide
Uphando kunye nophuhliso lwamachiza e-peptide lukhula ngokukhawuleza kumayeza.Nangona kunjalo, uphuhliso lwamachiza e-peptide lulinganiselwe ngeempawu zabo.Ngokomzekelo, ngenxa yobuntununtunu obukhethekileyo kwi-hydrolysis ye-enzymatic, ukuzinza kuyancipha, kwaye ukuhluka kwe-steric conformation kubangela ukuchasana okujoliswe kuyo okuphantsi, i-hydrophobicity ephantsi, kunye nokungabikho kwenkqubo ethile yokuthutha.Ukuze woyise ezi peptides, izisombululo ezininzi ezicetywayo kunye nokusetyenziswa ngempumelelo kolunye uhlobo lwepeptide yenye yazo.
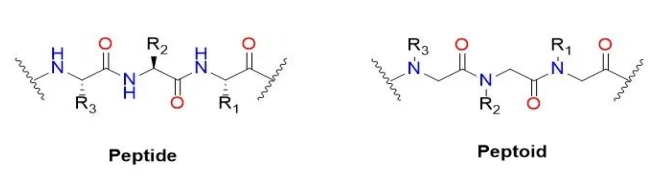
Uhlobo lwe-peptide (igama lesiNgesi: i-Peptoid) okanye i-Poly - N - endaweni ye-glycine (igama lesiNgesi: i-Poly real - N - substitutedglycine), yi-quasi peptide compounds of peptide in the main chain.Itsheyina elisecaleni lekhabhoni yealpha lihambisa initrogen yekhonkco eliphambili endaweni yekhonkco elisecaleni.Kwi-polypeptide yasekuqaleni, iqela le-R le-amino acid side chain limele i-20 eyahlukeneyo ye-amino acids, kodwa iqela le-R linokhetho oluninzi kwi-peptoid.Kwi-peptide, i-peptide kwikhonkco eliphambili le-amino acids kwi-alpha carbon nitrogen endaweni yokudluliselwa kwekhonkco elisecaleni kwikhonkco eliphambili.Kuyafaneleka ukukhankanya ukuba i-peptides ngokubanzi ayivelisi izakhiwo eziyalelwe kwinqanaba eliphezulu njengezakhiwo zesibini kwi-peptides kunye neeprotheni ngenxa yokungabikho kwe-hydrogen kwi-nitrogen backbone.Injongo yokuqala yePeptide kukuphuhlisa i-peptide ezinzileyo kunye neprotease yamachiza amancinci emolekyuli.
Uhlalutyo lweendlela zokuhlanganisa i-peptide
Indlela ye-peptide synthesis yaziswa
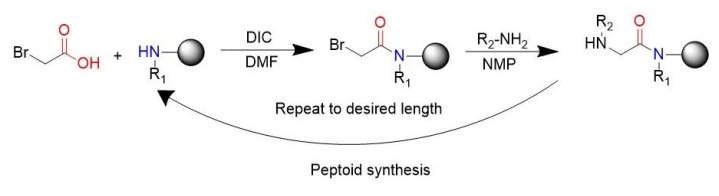
Indlela yokudityaniswa kwe-peptide ethandwa ngokubanzi yindlela yokudityaniswa kwe-subsingle eyakhiwe nguRonZuckermann, ngalinye lahlulwe ngamanyathelo amabini: i-acylation kunye nokufuduswa.Kwi-acylation, inyathelo lokuqala kukwenza i-haloacetic acid isebenze kunye ne-amines eseleyo ekupheleni kwesinyathelo sangaphambili, ngokuqhelekileyo i-disopropyl carbonized diimine.I-bromoaceticacid yenziwe yasebenza nge-disopropylcarbodiimide."Kwi-substitution reactions (i-bimolecular nucleophilic substitution reactions), i-amine, eyona nto iphambili, ihlasela enye i-halogen ukuze yenze i-N-substituted glycine."Indlela yokwenziwa kwe-subunitary isebenzisa ii-amine eziphambili ezifumaneka lula ukuvelisa iipeptides, ngaloo ndlela ivumela ukuhlanganiswa kwemichiza yeepeptides.
Ulwandiso oluluqilima kwi-peptide synthesis yeklasi lunamava atyebileyo, lunokukunika iintlobo ngeentlobo zenkonzo ye-peptide synthesis.
Uhlalutyo lweendlela zokuhlanganisa i-peptide
Inzuzo yepeptide enjalo
Izinzile ngakumbi: iipeptoids zizinzile kwi-vivo kune-peptides.
Ukukhetha ngakumbi: I-Peptoids ifaneleka kakuhle kwizifundo ezidityanisiweyo zokufunyanwa kweziyobisi kuba iindidi ezininzi ezahlukeneyo zebhloko zokwakha ze-polypeptide zinokufunyanwa ngokuguqulwa kweqela le-amino lomqolo.
Okusebenzayo ngakumbi: Ubuninzi bezakhiwo ze-peptoid bunokwenza ukuba i-peptoid ibe lukhetho olulungileyo lwendlela yokuskena ukufumana ngokukhawuleza izakhiwo ezithile ezibophelela kwiiproteni.
Amandla okuthengisa amaninzi: iimpawu zohlobo lwepeptide mazibe luhlobo lophuhliso lwechiza olunokubakho kakhulu.
Ixesha lokuposa: Dec-07-2023